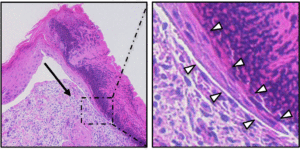
Maintaining proper tissue homeostasis in the skin involves carefully balancing cellular proliferation and differentiation, and responding to environmental insults such as wounding. An essential step in epidermal wound healing is the migration of keratinocytes across the wound bed to re-form the barrier. Chronic ulcers, due to diseases such as diabetes, lack proper control of the re-epithelialization process, which keeps wounds from healing properly. In the Ramsey laboratory, we are exploring the molecular mechanisms that control the migration of keratinocytes during epidermal wound healing, with the goal of developing therapies to alleviate chronic ulceration.
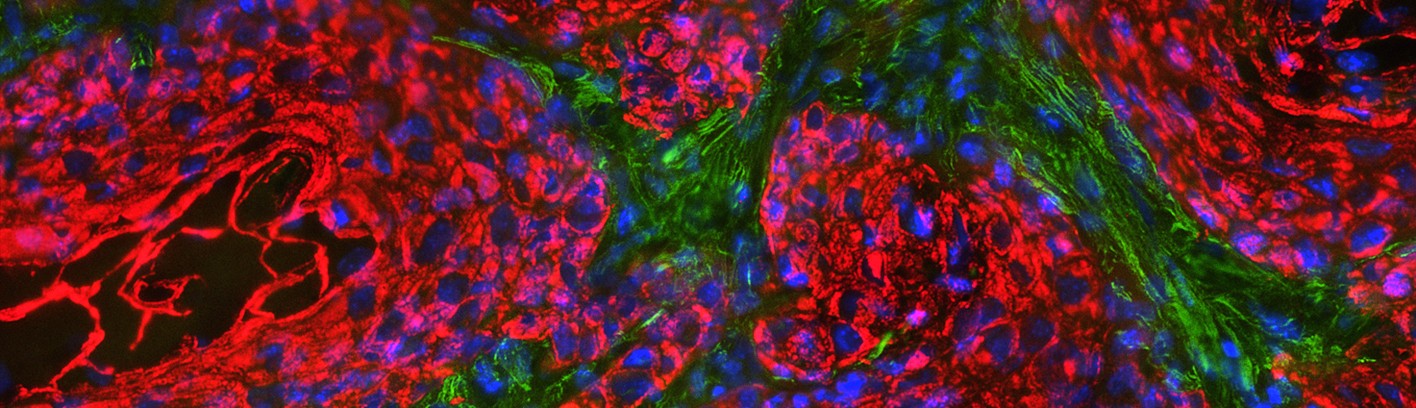
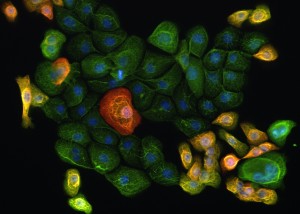
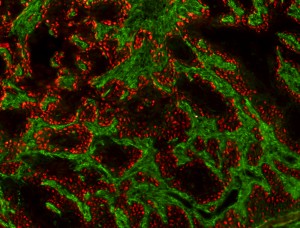
Squamous Cell Carcinoma (SCC) is a common cancer that develops in stratified epithelial tissues such as the epidermis, the oral cavity, and the lungs. The epidermis is the most common site of SCC, and cutaneous SCC that is not cured by surgery has few alternative treatments, resulting in approximately 9000 deaths per year. In addition it has been difficult to identify cutaneous SCC tumors that will eventually recur and metastasize. SCC tumors found in the head & neck region and the lungs have much poorer prognosis, as surgical options are often limited. Despite intense study, there are still few effective treatments for SCC patients, in part due to the lack of molecular understanding of the disease.
The Ramsey laboratory is focused on exploring transcriptional drivers of SCC, in order to develop more targeted and effective therapies. Using tissue-specific activation of driving oncogenes combined with inactivation of tumor suppressors, we are working to develop pre-clinical SCC models that reflect genetic alterations found in Human SCC to test novel therapeutics and elucidate how specific alterations promote tumorigenesis. To complement this work, we have also developed a variety of biochemical systems to investigate the role of transcription factors and associated co-factors in driving SCC-specific transcription. These tools can be applied to study the large variety of transcription factors that contribute to the pathogenesis of SCC. Understanding the differences in transcriptional networks between normal and cancerous tissues will offer insight into the vulnerabilities of SCC tumors that can be therapeutically targeted to improve the survival and quality of life of patients.